Calculation of Decay Activity of One Gram of Natural Uranium
Photo: Blocks of uranium metal produced for the Manhattan project in the 1940s. The smallest cubes each have a mass of about 40 grams. Courtesy of the U. S. Department of Energy.
Problem
How many atoms decay each second in a 1.000 gram sample of natural uranium? Solution
Short answer: 12,000 atoms per second (12 kBq) if you consider uranium-238 alone; 25,000 atoms per second (25 kBq) if you consider all the uranium isotopes present in natural uranium; or 50,000 atoms per second (50 kBq) if you also consider the decay products that accumulate over the course of a few months or longer following extraction from the uranium ore.Detailed Calculation
Natural uranium consists of the following isotopes: | Uranium isotope |
Percentage |
Half-life |
| uranium-238 |
99.275% |
4.47 billion years |
| uranium-235 |
0.720% |
704 million years |
| uranium-234 |
0.00548% |
245 thousand years |
Note: The numbers don't add up to exactly 100 percent due to rounding.
Let's consider the uranium-238 by itself first, since it makes up more than 99% of natural uranium.
Decay of uranium-238 alone
The 1.000 gram of uranium contains 0.993 gram of uranium-238. Uranium-238 has a half-life of 4.47 billion years. Let's convert that to seconds:
(4.47 x 109 years) x (365.25 days/year) x (24 hours/day) x (60 minutes/hour) x (60 seconds/minute) = 1.411 x 1017 seconds
The get the mean lifetime of a radioactive isotope, designated τ (Greek letter tau), divide the half-life by the natural logarithm of 2:
mean lifetime τ = half-life / ln(2) = (1.411 x 1017 seconds) / 0.6931 = 2.035 x 1017 seconds
The decay rate, designated λ (Greek letter lambda), is the fraction of the total mass that decays in one unit of time. It is equal to the inverse of the mean lifetime:
decay rate λ = 1/τ = 1 / (2.035 x 1017 seconds) = 4.914 x 10-18 per second
This is the fraction of the uranium-238 that decays in one second, so a mass of 0.993 gram of uranium decays at a rate of 0.993 x 4.914 x 10-18 = 4.88 x 10-18 gram per second. To convert this decay rate from grams per second to atoms per second, you use the atomic mass of uranium-238, 238 grams per mole, and the Avogadro constant, 6.022 x 1023 atoms per mole:
(4.88 x 10-18 gram / second) x (1.0 mole / 238 grams) x (6.022 x 1023 atoms / mole) = 1.23 x 104 atoms/second
This is 12,300 atoms/second or 12,300 becquerels (12.3 kBq)
Decay rate of uranium-235 alone
The 1.000 gram of uranium contains 0.0072 gram of uranium-235. Uranium-235 has a half-life of 704 million years. Let's convert that to seconds:
(704 x 106 years) x (365.25 days/year) x (24 hours/day) x (60 minutes/hour) x (60 seconds/minute) = 2.22 x 1016 seconds
mean lifetime τ = half-life / ln(2) = (2.22 x 1016 seconds) / 0.6931 = 3.205 x 1016 seconds
decay rate λ = 1/τ = 1 / (3.205 x 1016 seconds) = 3.12 x 10-17 per second
The decay rate of 0.0072 gram of uranium-235 is:
(0.0072 gram) x (3.12 x 10-17 per second) x (1.0 mole / 235 grams) x (6.022 x 1023 atoms / mole) = 570 atoms/second
This is 570 atoms/second or 570 becquerels.
Decay rate of uranium-234 alone
The 1.000 gram of uranium contains 5.48 x 10-5 gram of uranium-234. Uranium-234 has a half-life of 245 thousand years. Let's convert that to seconds:
(245 x 103 years) x (365.25 days/year) x (24 hours/day) x (60 minutes/hour) x (60 seconds/minute) = 7.73 x 1012 seconds
mean lifetime τ = half-life / ln(2) = (7.73 x 1012 seconds) / 0.6931 = 1.115 x 1013 seconds
decay rate λ = 1/τ = 1 / (1.115 x 1013 seconds) = 8.97 x 10-14 per second
The decay rate of 5.48 x 10-5 gram of uranium-235 is:
(5.48 x 10-5 gram) x ( 8.97 x 10-14 per second) x (1.0 mole / 234 grams) x (6.022 x 1023 atoms / mole) = 12,600 atoms/second
This is 12,600 atoms/second or 12,600 becquerels (12.6 kBq)
Decay rate of natural uranium per gram
The total decay activity for the three uranium isotopes (U-238, U-235, and U-234) in one gram of natural uranium is: 12.3 kBq + 0.570 kBq + 12.6 kBq = 25.5 kBq
Note: Your answer might be slightly different due to rounding errors.
Relationship between uranium-238 and uranium-234 decay rates
You might be surprised to find that nearly half of the decay activity
comes from uranium-234, which makes up only 1/200 of 1% of the total
amount of uranium. The reason is that it has a much shorter half-life,
just 245,000 years compared to 4.5 billion years for uranium-238, so it
decays much faster. You might also notice the similar decay rates of uranium-238 and uranium-234, both around 12 kBq per gram of natural uranium. This is not a coincidence. Uranium-234 exists in nature only because it is a product in the decay chain of uranium-238. A gram of uranium-238 decays at a rate of 12 kBq, producing thorium-234 at a rate of 12 kBq, which decays into protactinium-234, which decays into uranium-234, and so on. Each nuclide in the decay chain accumulates to an equilibrium concentration such that it is produced at, and decays away at, the same rate as the ultimate ancestor nuclide, uranium-238. (The results obtained in the calculations above are slightly different due to rounding errors.)
Uranium-235, on the other hand, is a primordial nuclide like uranium-238. It exists in nature because is was created when the Earth was formed and has a long enough half-life that it still exists today, 4.5 billion years later. There is no particular relationship between the decay rates of uranium-235 and uranium-238. Because uranium-235 has a shorter half -life than uranium-238, its relative concentration in natural uranium decreases over geological time spans.
Decay of uranium decay-chain products
The answer obtained earlier for the activity of natural uranium, about
25 kBq per gram, is correct for natural uranium freshly
extracted from uranium ore. However, that's not the end of the story. As
uranium decays, its decay products begin to accumulate and they themselves
begin to decay, thereby increasing the total activity of the material
significantly, even after just a few weeks. Let's take a look at the primary decay products and half-lives of the first few nuclides in the uranium-238 decay chain:
| Nuclide |
Half-life |
Decay product |
| uranium-238 |
4.47 billion years |
thorium-234 |
| thorium-234 |
24.1 days |
protactinium-234 |
| protactinium-234 | 1.16 minutes |
uranium-234 |
| uranium-234 |
245,000 years | thorium-230 |
| thorium-230 |
75,400 years |
radium-226 |
| radium-226 |
1,600 years |
radon-222 |
| ... and so on ... |
||
In undisturbed natural uranium ore, before the uranium is extracted, all of the decay-chain products of uranium-238 have accumulated to their equilibrium concentrations over geological time periods, such that they are all are being produced, and are decaying away, at the same rate, as determined by the amount and half-life of the ultimate ancestor nuclide, uranium-238. The equilibrium quantity of each nuclide is inversely proportional to its half-life.
Uranium-238 Decay-Chain Nuclides in Uranium Ore (Containing 1 g Uranium)
| Nuclide |
Half-life |
Quantity present |
Decay rate |
| uranium-238 |
4.47 billion years |
1/238 mole |
12.3 kBq |
| thorium-234 |
24.1 days |
1/238 mole x (1.48 x 10-11) |
12.3 kBq |
| protactinium-234 | 1.16 minutes |
1/238 mole x (4.93 x 10-16) | 12.3 kBq |
| uranium-234 |
245,000 years | 1/238 mole x (5.48 x 10-5) | 12.3 kBq |
| thorium-230 |
75,400 years |
1/238 mole x (1.69 x 10-5) | 12.3 kBq |
| radium-226 |
1,600 years |
1/238 mole x (6.53 x 10-7) | 12.3 kBq |
| ... and so on ... |
|||
In the Quantity present column above, the number in parentheses is the half-life of the nuclide divided by the half-life of the ultimate ancestor nuclide, uranium-238. For example, the amount of thorium-230 present under equilibrium conditions is 1.0/238 mole (the amount of the ancestor uranium-238 present) multiplied by the ratio of the two half-lives (75,400 / 4,470,000,000 = .0000169).
When uranium is extracted chemically from the ore, all the uranium isotopes are retained, and all the decay products are left behind in the waste product, the mill tailings. The following table shows what the extracted uranium contains (not including the uranium-235, which we will consider later).
Uranium-238 Decay-Chain Nuclides in 1 g Freshly Extracted Uranium
| Nuclide |
Half-life |
Quantity present |
Decay rate |
| uranium-238 |
4.47 billion years |
1/238 mole |
12.3 kBq |
| thorium-234 |
24.1 days |
none |
none |
| protactinium-234 | 1.16 minutes |
none |
none |
| uranium-234 |
245,000 years | 1/238 mole x (5.48 x 10-5) | 12.3 kBq |
| thorium-230 |
75,400 years |
none |
none |
| radium-226 |
1,600 years |
none |
none |
| ... and so on ... |
|||
However, thorium-234 immediately begins to accumulate as the decay product of uranium-238 and itself begins to decay. Because it is being produced at a constant rate (12,300 atoms per second), and its own decay rate is proportional to the amount present, it accumulates to one-half its equilibrium amount in one half-life, to three-fourths of that amount in two half-lives, to seven-eights of that amount in three half-lives, and so on.
Therefore, after one half-life (24 days), we have the following amounts of uranium-238 decay-chain products.
Uranium-238 Decay-Chain Nuclides 24 Days After Extraction of 1 g Uranium
| Nuclide |
Half-life |
Quantity present |
Decay rate |
| uranium-238 |
4.47 billion years |
1/238 mole |
12.3 kBq |
| thorium-234 |
24.1 days |
1/238 mole x (1.48 x 10-11) x 0.5 |
6.1 kBq |
| protactinium-234 | 1.16 minutes |
1/238 mole x (4.93 x 10-16) x 0.5 |
6.1 kBq |
| uranium-234 |
245,000 years | 1/238 mole x (5.48 x 10-5) | 12.3 kBq |
| thorium-230 |
75,400 years |
negligible |
none |
| radium-226 |
1,600 years |
none | none |
| ... and so on ... |
|||
Protactinium-234 has a half-life of one minute, so it decays just about as fast as it is produced. So it also reaches one-half of its equilibrium amount in 24 days, because it is being produced at one-half its equilibrium rate. Thorium-230 is also accumulating at 12,300 atoms per second as a decay product of uranium-234. However, because thorium-230 has such a long half-life on a human time scale (75,400 years), it can be considered, for practical purposes, a stable element that accumulates without decay. As a result, none of the decay-chain nuclides below thorium-230, such as radium and radon, will be produced in the next several tens of thousands of years.
By the time a few months have passed, thorium-234 and protactinium-234 accumulate to their equilibrium amounts and decay at the same equilibrium rate of 12.3 kBq.
Uranium-238 Decay-Chain Nuclides Beyond a Few Months After Extraction of 1 g Uranium
| Nuclide |
Half-life |
Quantity present |
Decay rate |
| uranium-238 |
4.47 billion years |
1/238 mole |
12.3 kBq |
| thorium-234 |
24.1 days |
1/238 mole x (1.48 x 10-11) |
12.3 kBq |
| protactinium-234 | 1.16 minutes |
1/238 mole x (4.93 x 10-16) |
12.3 kBq |
| uranium-234 |
245,000 years | 1/238 mole x (5.48 x 10-5) | 12.3 kBq |
| thorium-230 |
75,400 years |
negligible |
none |
| radium-226 |
1,600 years |
none | none |
| ... and so on ... |
|||
Therefore, total activity of uranium-238 and uranium-234, as long as it has been sitting around for a few months or more after extraction from the ore, has an activity of 4 x 12.3 kBq = 49.2 kBq.
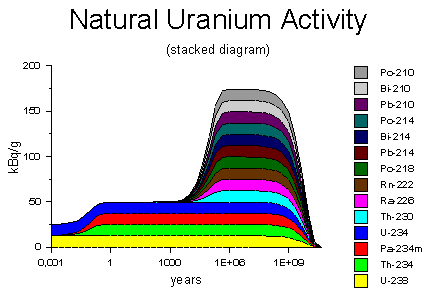 The diagram on the right shows the decay activity of one gram of natural
uranium on the logarithmic time scale, ranging from 3 days to 30 billion
years. When freshly extracted from the ore, the uranium has an decay
rate of 25 kBq, arising equally from the uranium-238 and uranium-234. Within a
few months, the decay products thorium-234 and protactinium-234 accumulate to their
equilibrium concentrations, doubling the activity to 50 kBq. The diagram on the right shows the decay activity of one gram of natural
uranium on the logarithmic time scale, ranging from 3 days to 30 billion
years. When freshly extracted from the ore, the uranium has an decay
rate of 25 kBq, arising equally from the uranium-238 and uranium-234. Within a
few months, the decay products thorium-234 and protactinium-234 accumulate to their
equilibrium concentrations, doubling the activity to 50 kBq.Diagram source: WISE Uranium Project, Uranium Radiation Properties |
Meanwhile, the small amount of uranium-235 in natural uranium also produces a nuclide that contributes to the total activity. A similar consideration of the uranium-235 decay-chain produces the following results.
Uranium-235 Decay-Chain Nuclides Beyond a Few Days After Extraction of 1 g Uranium
| Nuclide |
Half-life |
Quantity present |
Decay rate |
| uranium-235 |
704 million years |
0.0072 / 235 mole |
570 Bq |
| thorium-231 |
25.52 hours |
0.0072 / 235 mole x (4.1 x 10-12) | 570 Bq |
| protactinium-231 | 32,800 years |
negligible |
none |
| actinium-227 |
21.8 years |
none | none |
| thorium-227 |
18.7 days |
none |
none |
| radium-223 |
11.4 days |
none | none |
| ... and so on ... |
|||
Thus, the activity of one gram of natural uranium due to the presence of uranium-235 that has been sitting around for a few days or more is 2 x 570 Bq = 1,140 Bq = 1.14 kBq.
The final answer
The total activity of one gram of natural uranium that has been sitting
around for a few months or longer is the activity of uranium-238 and its
decay products thorium-234, protactinium-234, and uranium-234 (49.2
kBq); plus the activity of the 0.72% of uranium-235 present and its
decay product thorium-231 (1.1 kBq). Thus, the total activity is 50.3 kBq per gram of natural uranium. Why is the decay of uranium isotopes important?
Shortly after the discovery of radioactivity by Henri Becquerel in 1896, Marie Curie
found that only uranium and thorium were radioactive among the known
elements. She also found that the form of uranium, whether pure metal,
uranium salt, or uranium oxide, made no difference in the strength of
the radioactivity. Only the amount of uranium present was important,
with one exception -- pitchblende ore was four times more radioactive
than expected for its uranium content. Curie correctly hypothesized that the extra radioactivity was caused by the presence of undiscovered radioactive elements, present in small quantities but with greater activity that uranium. By separating pitchblende ore into is constituent parts, she discovered two new elements, polonium and radium, which are decay-chain products of uranium-238.
In assessing the environmental dangers of uranium mining, one must consider not only the uranium extracted from the ore, but also the leftover waste rock left behind after extraction, the mill tailings, which are more radioactive than the uranium itself. For more information on this subject, see Peter Diehl's Uranium Radiation Properties web page and its top-level index page, the World Information Service on Energy Uranium Project.
Among the isotopes of natural uranium, only uranium-235 is fissile, that is, can be used in nuclear reactors or to make nuclear weapons. For these purposes, the uranium must be enriched from the natural 0.72% concentration to at least 3% for nuclear fuel or at least 90% for nuclear weapons.
Because all isotopes of uranium are chemically identical, the only way to separate out the uranium-235 from uranium-238 is to take advantage of the small difference in mass of the two nuclides. In the modern gas centrifuge method, a gaseous compound of uranium is spun in a rapidly rotating cylinder, causing the heavier uranium-238 molecules to accumulate at the outside, forcing the lighter uranium-235 molecules to the inside.
The uranium from which most of the uranium-235 isotope has been removed is called depleted uranium, which has several practical uses but can be dangerous because it is radioactive, and the radioactivity grows over time. It is also toxic in the same way as lead or cadmium.
